World Breastfeeding Week 2020 has sparked conversations about the broader environmental and social impacts of infant feeding decisions.
In our research, we have observed a trend of increased availability and promotion of breast-milk substitute products for young children. Beyond the carbon footprint generated in the production, packaging and distribution of a broader range of breast-milk substitutes, nutrition experts have raised health concerns about these so-called “growing-up milks.”
Helen Keller International’s ARCH Project recently assessed the sugar content listed on labels of growing-up milk (GUM)* products available for sale in Indonesia. Our team also evaluated the nutrient profiles of these products and compared the labels with local and international standards including the Codex Draft Revised Standard for Follow-up Formula (CXS 156-1987) and the International Code of Marketing of Breast-milk Substitutes. Our findings revealed almost three quarters (70%) of GUMs assessed have high sugar levels which classify them as unhealthy according to the UK Food Standards Agency Nutrient Profiling Method, leading to questions about whether these products are appropriate for feeding children between 12-36 months as indicated on the labels. Our recently published report also indicates that GUMs in Indonesia are nine times more expensive than cow’s milk, which is an appropriate nutrient-dense drink for non-breastfed children over 12 months of age.
Lack of regulation of GUM product claims and labeling practices are cause for concern and can lead to misinformation and the overall promotion of inappropriate child feeding. These findings from Indonesia provide insights into the marketing of GUMs in a specific context as well as reflect international trends of unethical marketing of breast-milk substitutes.
These findings from Indonesia, published here will provide insights into the marketing of growing-up milks in a specific context. However, the proliferation of these products reflects international trends and a long history of unethical marketing of breastmilk substitutes:
Timeline of key events in the history of breastmilk substitute production and marketing (from Changing Markets Foundation)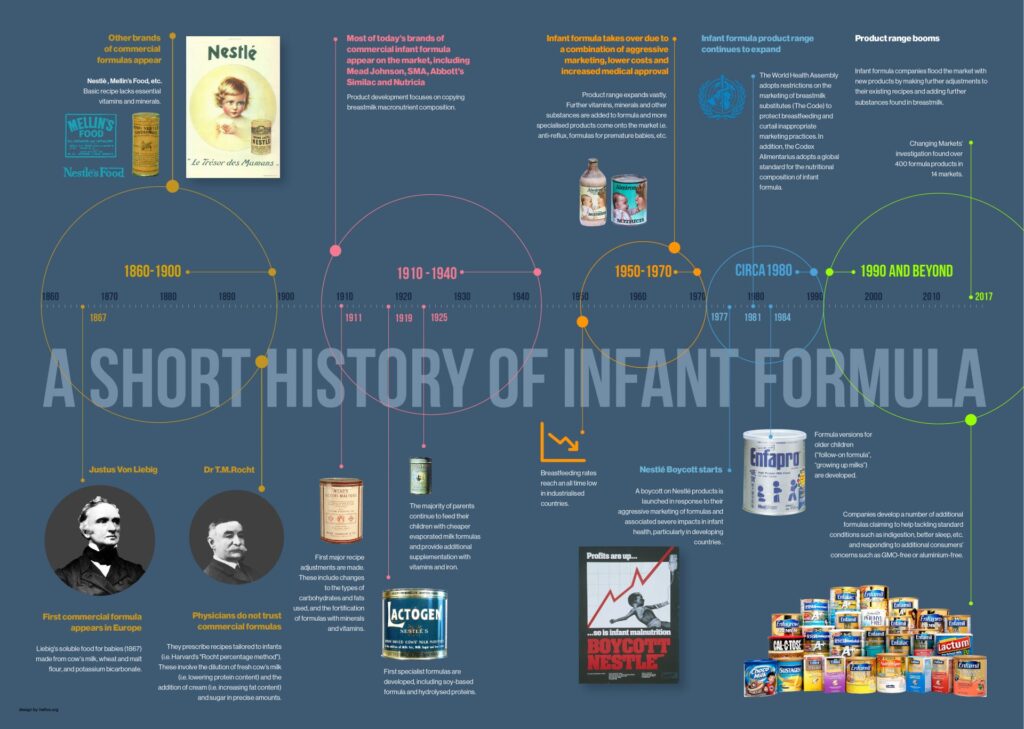
Marketing portrays GUMs as the next step for children using infant formula, leading to consumer confusion
GUMs are increasingly marketed as essential parts of entire child nutrition product lines. These products have been sold under a variety of names, including “follow-up formulas,” “toddler formulas”, and “growing-up milks.” Since the 1980s, these products have become a prominent and growing segment of the approximately $70 billion USD breastmilk substitute (BMS) industry.1 They are labelled similarly to infant formula products and positioned as sequential steps for child feeding from birth through the first few years of life.2
Similar color schemes, logos, and marketing copy appear on labels of brands’ breastmilk substitutes, with different product names or stages usually appearing in smaller lettering to indicate the appropriate age of introduction. This practice, along with cross-promotion of GUMs and infant formulas observed in retail sales promotions, has been criticized as an indirect means of promoting breastmilk substitutes for earlier age ranges without contravening national regulations.3–5 Also of concern is the potential misuse of GUMs due to ambiguity in labeling and branding, especially since GUMs are usually priced to be cheaper than infant formulas. GUMs are not nutritionally sufficient for infants in the first year of life, but evidence suggests that caregivers perceive them as interchangeable with infant formula.6–8
Examples of cross-promotion of infant formula through similar branding elements on growing-up milk labels2
Companies market GUMs aggressively, claiming unfounded health benefits
In addition to traditional promotional channels (like print advertisements, point-of-sale displays, billboards, posters, etc.), GUMs are also marketed to consumers through in-person company representatives and promotional events in many parts of the world.9,10 They are also included in longstanding and controversial health facility-based breast-milk substitute marketing strategies such as distribution of branded discharge packs for new mothers11,12 and provision of financial incentives to health workers.13,14 Breast-milk substitute companies also engage in sponsorship of national and regional pediatric organizations, a practice which presents conflicts of interest and provides another avenue for indirect promotion by encouraging brand loyalty among care providers.15 Online breast-milk substitute brand and product promotion is also becoming common, through interactive web pages, social media presences, and sponsored posts on parenting sites in addition to e-commerce activities.16
Common claims of GUMs include improved cognitive development, greater intelligence, weight management, better immunity and improved digestion.17–19 These claims are inconsistently supported by scientific evidence, but a solid evidence base is not required by the Codex Alimentarius Standard for Follow-up Formula to back these claims.20–22 GUMs are also positioned as solutions for picky toddlers to get more nutrients into their diet,23 which is often achieved through added sweeteners and flavorings designed to appeal to young children.24
Nutrition experts uphold that for children over 12 months of age, whole cow’s milk is an appropriate substitute for breastmilk.25 Commercial GUMs do not provide necessary nutrients beyond what can be achieved through complementary diets in most settings. Use of GUMs may also displace continued breastfeeding in the diets of toddlers, who can still benefit from the reduced morbidity, mortality, and risk of childhood obesity associated with longer breastfeeding durations.26,27
Comparison of key nutrients between growing-up milk and cow’s milk24
| Brand and product | NPI score | Calories (kcal) | Sat fat (g) | Total sugar (g) | Sodium (mg) | Fiber (g) | Protein (g) |
| Happy Tot Grow & Shine | 72 | 68.2 | 0.0 | 2.9 | 24.3 | 0.0 | 1.9 |
| Milk, low-fat, fluid, 1% milkfat | 72 | 42.0 | 0.6 | 5.2 | 44.0 | 0.0 | 3.4 |
| Nido Fortificada (Fortified) | 70 | 59.1 | 1.8 | 4.1 | 38.8 | 0.0 | 2.2 |
| Gerber Good Start Grow | 70 | 63.3 | 0.2 | 4.9 | 24.3 | 0.0 | 1.9 |
| Milk, reduced-fat, fluid 2% milkfat | 70 | 50.0 | 1.3 | 5.1 | 47.0 | 0.0 | 3.3 |
| Nido Kinder 1+ | 70 | 70.2 | 0.9 | 5.5 | 35.1 | 0.4 | 2.2 |
| Milk, whole | 68 | 61.0 | 1.9 | 5.1 | 43.0 | 0.0 | 3.2 |
| Enfagrow Toddler Next Step | 68 | 75.0 | 1.2 | 5.2 | 37.5 | 0.4 | 2.8 |
| Go & Grow by Similac ** | 68 | 55.2 | 0.0 | 5.5 | 16.6 | 0.3 | 1.5 |
| Milk, chocolate, reduced-fat | 66 | 78.0 | 1.2 | 9.6 | 66.0 | 0.7 | 3.0 |
| Milk, chocolate, whole | 62 | 83.0 | 2.1 | 9.5 | 66.0 | 0.8 | 3.2 |
| Enfagrow Toddler Transitions Soy | 60 | 58.7 | 6.9 | 1.1 | 21.1 | 0.0 | 1.9 |
| Enfagrow Toddler Transitions | 58 | 58.7 | 6.3 | 1.4 | 21.1 | 0.0 | 1.5 |
| Enfagrow Toddler Transitions Gentlease | 58 | 58.7 | 6.2 | 1.4 | 23.5 | 0.0 | 1.5 |
*Per 100 grams **All products have the same nutrient content. Source: 2016 Rudd Center product analysis
While breastfeeding is recognized by The World Health Organization (WHO), UNICEF, and countess other experts as the best way to feed an infant, there will continue to be cases where families need or want an alternative. The use of GUMs, which are both unnecessary and expensive, has the potential to displace healthy diets and put young children at risk for undernutrition and poor health later in life. As advertising efforts and consumption of GUMs continue to rise around the world, there is an increasing need to strengthen international and national regulations on the marketing and labeling of these products.
*The ARCH Project uses this term to refer to animal or alternative milk-based powdered, concentrated or ready to feed breast-milk substitutes for children from 12 to 36 months of age
References:
- Rollins NC, Bhandari N, Hajeebhoy N, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet [Internet] 2016 [cited 2016 Jan 30];387(10017):491–504. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26869576
- Pereira C, Ford R, Feeley AB, Sweet L, Badham J, Zehner E. Cross-sectional survey shows that follow-up formula and growing-up milks are labelled similarly to infant formula in four low and middle income countries. Matern Child Nutr [Internet] 2016 [cited 2017 Jan 12];12(S2):91–105. Available from: http://doi.wiley.com/10.1111/mcn.12269
- Helen Keller International. CCNFSDU 2019: Review of the Codex Standard for Follow-Up Formula [Internet]. 2019 [cited 2020 Apr 30];Available from: http://doi.org.10.1016/S0140-6736
- Berry NJ, Jones SC, Iverson D. Circumventing the WHO Code? An observational study. Arch Dis Child [Internet] 2012;97(4):320–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21719442
- World Health Organization. Information Note: Clarification on the classification of follow-up formulas for children 6-36 months as breastmilk substitutes. Geneva: 2018.
- Romo-Palafox MJ, Pomeranz JL, Harris JL. Infant formula and toddler milk marketing and caregiver’s provision to young children Expert recommendations, infant formula, marketing claims, policy, toddler milk. 2020 [cited 2020 May 1];Available from: https://doi.org/10.1111/mcn.12962
- Berry NJ, Jones S, Iverson D. It’s all formula to me: women’s understandings of toddler milk ads. Breastfeed Rev 2010;18(1):21–30.
- Cattaneo A, Pani P, Carletti C, et al. Advertisements of follow-on formula and their perception by pregnant women and mothers in Italy. Arch Dis Child [Internet] 2015 [cited 2016 Feb 26];100(4):323–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25512963
- Hadihardjono DN, Green M, Stormer A, Agustino, Izwardy D, Champeny M. Promotions of breastmilk substitutes, commercial complementary foods and commercial snack products commonly fed to young children are frequently found in points-of-sale in Bandung City, Indonesia. Matern Child Nutr 2019;15(S4).
- IBFAN. ICDC Focus: Aggressive Promotion- Growing-up milks [Internet]. Penang: 2016 [cited 2020 May 1]. Available from: www.ibfan-icdc.org
- Nelson JM, Li R, Perrine CG. Trends of US hospitals distributing infant formula packs to breastfeeding mothers, 2007 to 2013. Pediatrics 2015;135(6):1051–6.
- Walker M. Why Infant Formula Samples Pose a Risk to Health Care Providers, Hospitals, and Patients. J Obstet Gynecol Neonatal Nurs 2015;44(5):618–23.
- Wright CM, Waterston AJR. Relationships between paediatricians and infant formula milk companies. Arch Dis Child [Internet] 2006 [cited 2016 Aug 16];91(5):383–5. Available from: http://adc.bmj.com/cgi/doi/10.1136/adc.2005.072892
- McFadden A, Mason F, Baker J, et al. Spotlight on infant formula: coordinated global action needed. Lancet. 2016;387(10017):413–5.
- Grummer-Strawn LM, Holliday F, Jungo KT, Rollins N. Sponsorship of national and regional professional paediatrics associations by companies that make breast-milk substitutes: Evidence from a review of official websites. BMJ Open 2019;9(8):e029035.
- Abrahams SW. Milk and social media: online communities and the International Code of Marketing of Breast-milk Substitutes. J Hum Lact [Internet] 2012;28(3):400–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22674963
- Pereira C, Ford R, Feeley AB, Sweet L, Badham J, Zehner E. Cross-sectional survey shows that follow-up formula and growing-up milks are labelled similarly to infant formula in four low and middle income countries. Matern Child Nutr [Internet] 2016 [cited 2016 Apr 18];12 Suppl 2:91–105. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27061959
- Champeny M, Hou K, Diop EI, et al. Prevalence, duration, and content of television advertisements for breast milk substitutes and commercially produced complementary foods in Phnom Penh, Cambodia and Dakar, Senegal. Matern Child Nutr 2019;15(S4).
- Pomeranz JL, Romo Palafox MJ, Harris JL. Toddler drinks, formulas, and milks: Labeling practices and policy implications. Prev Med (Baltim) [Internet] 2018 [cited 2018 Apr 16];109:11–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29339115
- Changing Markets Foundation. Milking it-How milk formula companies are putting profits before science [Internet]. 2017 [cited 2020 Apr 30]. Available from: www.changingmarkets.org
- Hughes HK, Landa MM, Sharfstein JM. Marketing claims for infant formula the need for evidence. JAMA Pediatr. 2017;171(2):105–6.
- Codex Alimentarius Comission. STANDARD FOR FOLLOW-UP FORMULA CXS 156-1987 [Internet]. 2017 [cited 2018 Jun 19];Available from: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B156-1987%252FCXS_156e.pdf
- Turck D. Quelle place pour les laits « Croissance »? Arch Pediatr 2015;22(5):85–6.
- Harris JL, Fleming-Milici F, Frazier W, et al. Baby Food Facts 2016: Nutrition and marketing of baby and toddler food and drinks. 2016.
- World Health Organization. Guiding Principles for Feeding Non-Breastfed Children 6-24 Months of Age [Internet]. Geneva: 2005. Available from: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Guiding+principles+for+feeding+non-breastfed+children+6-24+months+of+age#0
- Grummer-Strawn LM, Mei Z. Does breastfeeding protect against pediatric overweight? Analysis of longitudinal data from the Centers for Disease Control and Prevention Pediatric Nutrition Surveillance System. Pediatrics [Internet] 2004 [cited 2020 May 1];113(2):e81-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14754976
- Sankar MJ, Sinha B, Chowdhury R, et al. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr [Internet] 2015 [cited 2020 May 1];104:3–13. Available from: http://doi.wiley.com/10.1111/apa.13147